Abstract
The treatment of Acute Leukemias worldwide predominantly relies on Standard of Care therapeutic schemes that have been established as the result of populational studies. However, such one-fits-all approach is associated with a high rate of refractory/relapsed patients for which is even more challenging to select sequential lines of therapies. It has therefore become clear that personalized approaches for therapy selection are necessary to improve outcomes, even more so when exploring later lines of treatment.
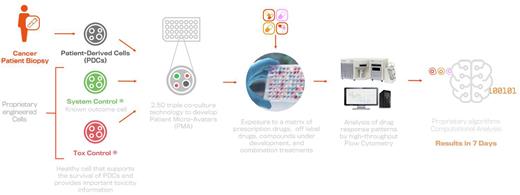
At OncoPrecision, we have developed a triple co-culture platform that mimics the tumor microenvironment and promotes the ex-vivo survival of Patient-Derived Cells (PDCs), allowing for predictions regarding the performance of the complete array of FDA-approved drugs, as well as experimental treatments, within 7 days. This pioneering technology, which we have named Patient Micro Avatars (PMAs), consists in the co-culture of PDCs with engineered neoplastic (System Control) and stromal (Tox Control) heterologous cells in combination with a multi-tagging approach coupled to high-throughput flow cytometry (See Figure). Our PMAs not only allow us to rank the activity of all approved treatments, but also unveil potential unspecific toxicities of novel treatments on non-tumoral cells, thus unlocking unique insights for early drug development programs.
In this work, we introduce OncoPrecision's PMA technology by presenting the clinical validation results from of an Observational Study performed in collaboration with seven healthcare centers from Argentina, which through approved IRBs have provided AML and ALL patient samples from peripheral blood and bone marrow, as well as clinical follow-up. We present ex-vivo testing results with a comprehensive AML drug matrix, which includes Standard of Care therapies, as well as experimental drugs and label expansion candidates. We illustrate the potential of PMAs to identify differential activity from several chemotherapy regimens, targeted therapies, and monoclonal antibodies in more than 30 patients, as well as complementary and mutually exclusive predictions when comparing to NGS panels from the same patient dataset.
Disclosures
Garcia:OncoPrecision: Current Employment, Current holder of stock options in a privately-held company, Honoraria, Research Funding. Buffa:OncoPrecision: Current Employment. Ridano:OncoPrecision: Consultancy, Honoraria. Scaglia:OncoPrecision: Current Employment. Nicola:OncoPrecision: Honoraria, Research Funding. Carbajosa:OncoPrecision: Current Employment. Garro:OncoPrecision: Current Employment, Research Funding. Arroyo:OncoPrecision: Current Employment, Honoraria. Conrrero:OncoPrecision: Current Employment, Research Funding. Fiorenza:OncoPrecision: Ended employment in the past 24 months, Honoraria. Alfonso:OncoPrecision: Honoraria, Research Funding. Bernaschini:OncoPrecision: Current Employment. Andino:OncoPrecision: Current Employment. Cavallo:OncoPrecision: Current Employment. Ferreira:OncoPrecision: Current Employment. Pavlovsky:Novartis, Pfizer, BMS, Pint Pharma: Speakers Bureau; Novartis, Pfizer: Membership on an entity's Board of Directors or advisory committees. Solimano:OncoPrecision: Consultancy, Honoraria. Zaki:OncoPrecision: Current Employment, Current holder of stock options in a privately-held company. Gatti:OncoPrecision: Current Employment, Current holder of stock options in a privately-held company. Llorens:OncoPrecision: Current Employment, Current holder of stock options in a privately-held company, Research Funding. Soria:OncoPrecision: Current Employment, Current holder of stock options in a privately-held company, Research Funding.
OffLabel Disclosure:
We will present some proof of concept examples of off-label drugs that show differential activity in a subset of AML patients to highlight the potential of our platform to characterize drugs under development and potential off-label applications of drug from other indications.
Author notes
Asterisk with author names denotes non-ASH members.